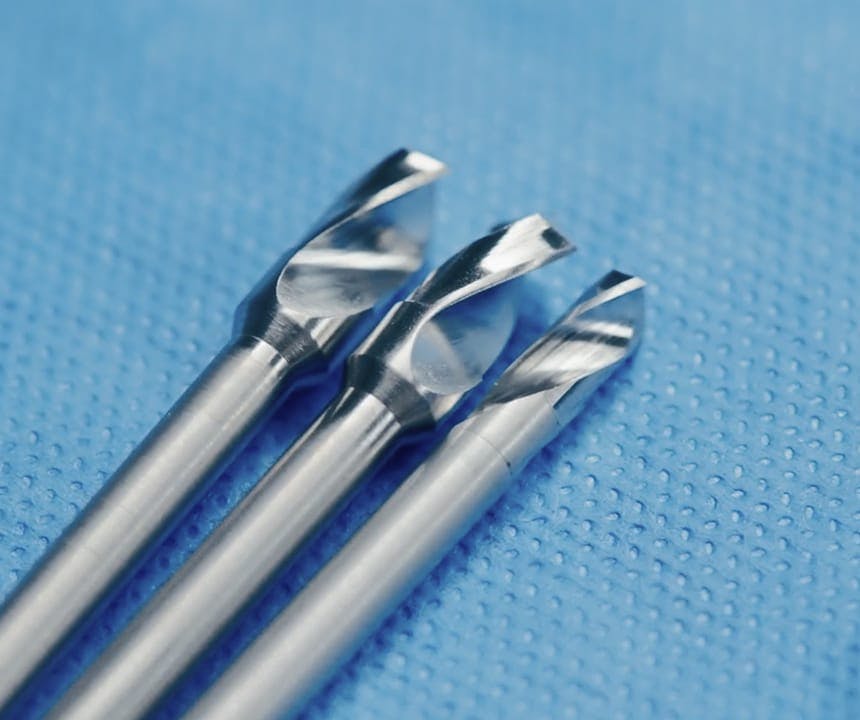
Precision access for Laser Interstitial Thermal Therapy procedures: safe, sterile, sharp, and ready to use.
Engineered for Precision and Protection
Every Phasor LITT-specialized Drill integrates Phasor’s anti-plunge safety system, mechanically preventing over-penetration while preserving the tactile control essential between cortical/cancellous/cortical layer transitions, for delicate cranial access with neurosurgical (rather than the heavier orthopedic drill borrowed for neurosurgery) design. The lightweight, balanced handpiece offers steady torque with minimal vibration, supporting safer use when trajectory really matters.
Safety and performance features:
- Adjustable anti-plunge stopper
- Single-use sterile drill eliminates cross-contamination risk
- Lightweight, powered driver reduces heat and vibration or trajectory deviation
- Fully pre-packaged and sterilized for immediate OR or MRI Suite use
- Compatible with leading frameless navigation systems including ROSA, Brainlab, Medtronic StealthStation, Stryker, and more
These LITT-specialized Phasor Drill’s design ensures every case begins with a sharp, sterile, and validated instrument, eliminating maintenance or resterilization-associated downtime and ensuring reproducible performance.